|
These kits are manufactured in accordance with the 98/79 EC directive for in vitro diagnostic devices. Only CE marked products can be used for diagnostic applications in Europe. |
 |
These kits are intended for in vitro diagnostic use. |
 |
These kits are for research use only and are not intended to be used for diagnostic procedures. |
 |
Federal Drug Administration, FDA validates diagnostic kits for in vitro diagnostic use in the United States. |
 |
Biological risk products. |
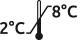 |
Storage between 2 and 8 ° C. |
 |
Reactive in liquid form. |
 |
Reactive in lyophilized form. |
 |
Reactive in frozen form. |
 |
Stability after opening at 2-8 ° C. |
 |
Products that can be refrozen. |
 |
Stability 12 months after refreezing at -20 ° C. |
 |
Manufacturer. |
 |
Importer |
 |
Distributor. |