HEMOSTASIS COAGULATION ROUTINE REAGENTS AUXILIARY REAGENTS BUFFERS, CACL₂, BSA
Citrate Sodium Chloride Buffer
Citrate Sodium Chloride buffer.
Dilution buffer for use in factor II,V, VII and X tests.
Documentation
Download the product sheetPrice list, safety data sheets and notices are accessible to our registered customers.



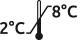

References
| 4-5400045 | Vial | 1 x 60 mL |














