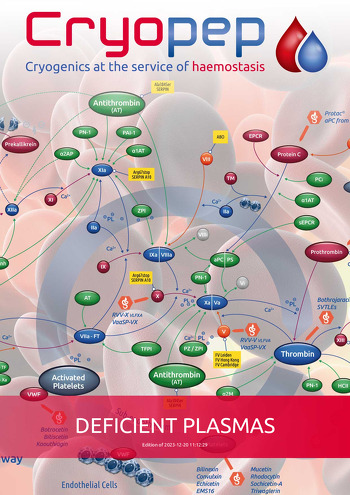
HEMOSTASIS COAGULATION RESEARCH DEFICIENT PLASMAS CONGENITAL DEFICIENT PLASMAS (BOTTLES)
Human Factor XII congenital deficicent plasma >5%





Plasma from a human donor with congenital FXII deficiency.
Packaging in bottle. The minimum packaged volume is 50 mL. The price offer is based on the volume requested.
| Reference | 6-PPD12C |
|---|---|
| Presentation | Vial |
| Format | Minimum 50 mL |
| Quote |
Price list, safety data sheets and notices are accessible to our registered customers.
| Reference | Presentation | Format | Quote | Product sheet |
|---|---|---|---|---|
| 6-PPD12C | Vial | Minimum 50 mL |